
ConnectEHR is a general purpose interoperability toolkit that works with your EHR to satisfy Interoperability requirements

Overview
ConnectEHR® is our ONC-certified, bolt-on software. We want to help you achieve ONC Certification—and go beyond—by providing easy-to-implement, interoperable functionality. ConnectEHR® offers a host of features to meet Certification, including support for USCDI, patient engagement and Interoperability goals, including drag-and-drop patient data views, data exports and a fully integrated FHIR API. ConnectEHR® also allows you to administer a customizable, multi-facility, and fully certified Patient Portal and FHIR API.
Can you create a 2.1 C-CDA using USCDIv2 or v3 data elements?
ConnectEHR generates and consumes C-CDA for 50+ EHR and Hospital Systems.
Features
- ONC Certified for 170.315(b)(1,7,8)(f)(1,2,5): Meets Promoting Interoperability and MACRA/MIPS requirements
- Supports C-CDAs: TOC, VDT, Batch, Care Plan, Referral Notes and Summary of Care, eCR, Reportable Labs
- HL7 Messages: Creates, receives and parses HL7 messages required for MU, including Public Health, Immunization and Lab results
- Secure Messaging: Integrates with MaxMD or any certified HISP for Direct secure messaging
View, Download, Transmit C-CDA Documents
170.315(e)(1),(b)(1)
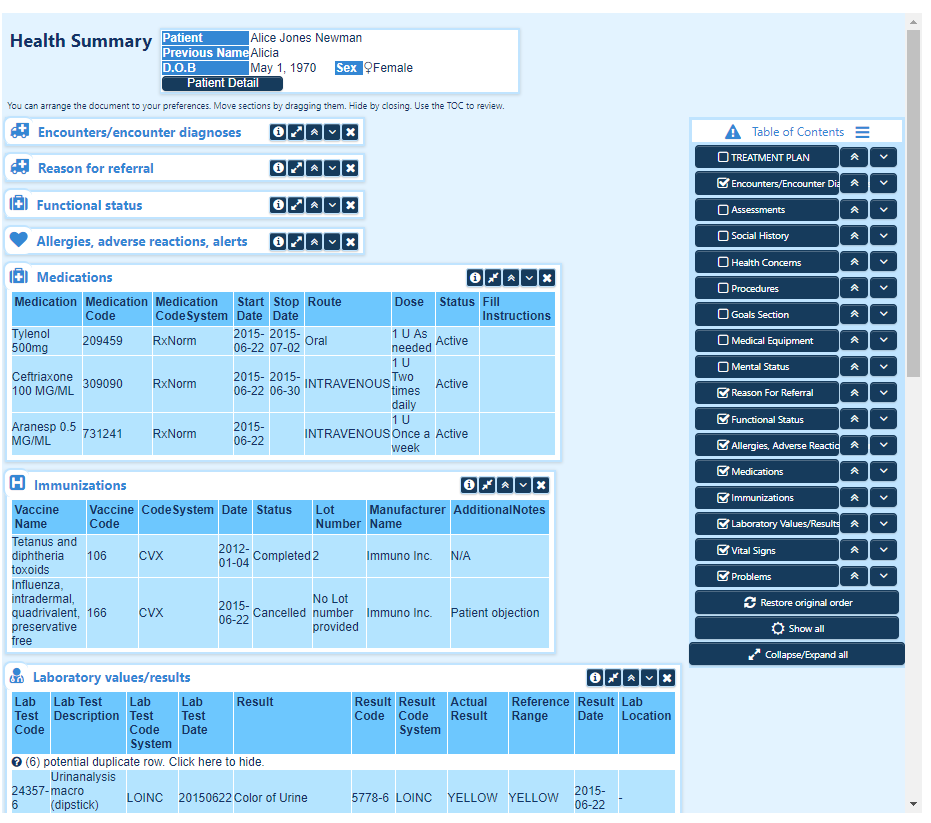
Supports Public Health Reporting
Dynamic’s eCR Gateway Workflow
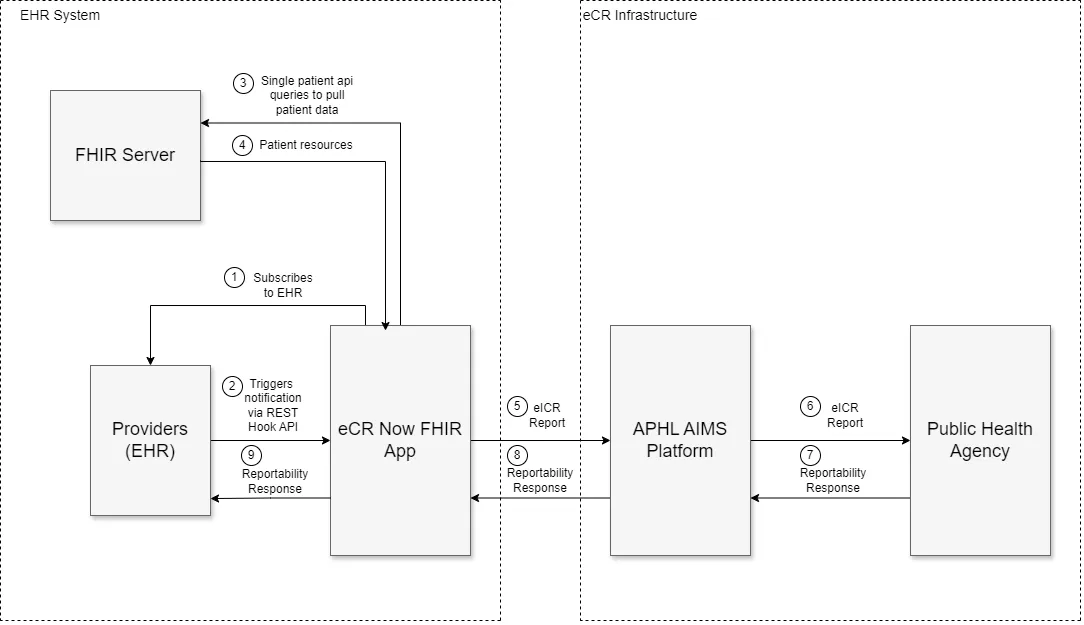
Information Blocking
ConnectEHR Added Payer Data to C-CDA
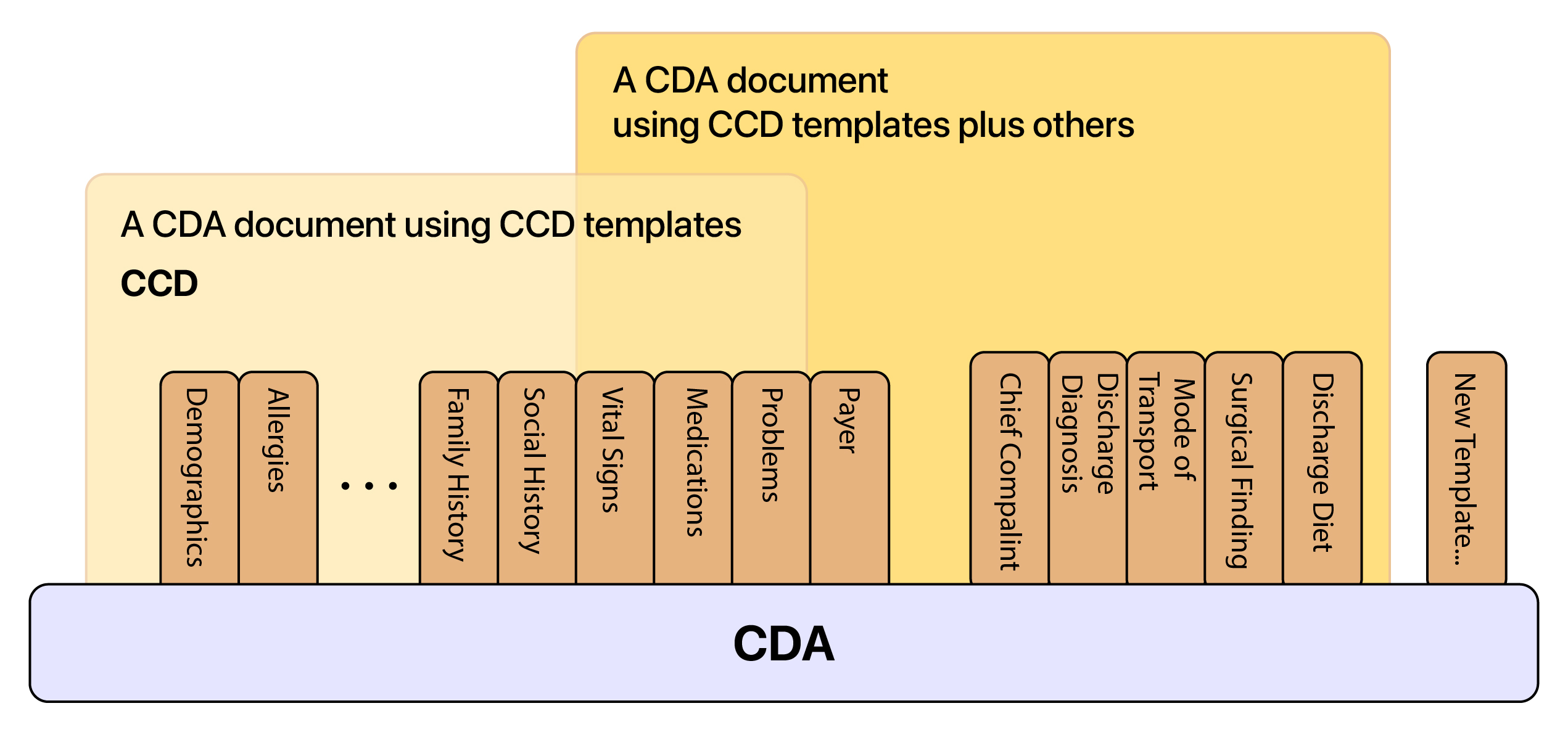
USCDI Supported Versions
The United States Core Data for Interoperability (USCDI) is a standardized set of health data classes and constituent data elements for nationwide, interoperable health information exchange.
A USCDI “Data Class” is an aggregation of various Data Elements under a common theme or use case. A USCDI “Data Element” is the most granular level at which a piece of data is represented in the USCDI for exchange.
Currently, DHIT supports USCDI versions 1, 2, and 3. Version 4 is currently slated for development.
USCDI V1
USCDI V2
USCDI V3
USCDI V4

Contact DHIT for payment instructions. When payment is complete, you will receive the password to download the software
